Short peptides and amino acids are signal molecules, which regulate significant physiological processes in human and animal organism in the norm and pathology. The goal of work is to investigate combined activity of short peptides and end amino acids in compositions of these peptides, on brain cortex, heart, liver, spleen and vessel organotypic tissue cells of young and old rats growth. In the presence of end amino acids in compositions of peptides the growth of brain cortex, liver, spleen and heart tissues increased in cultures, obtained from young animals. Peptides increased the index of growth area of these tissues in 20-22% and combined of amino acids and peptides increased these values on 31-41%. In the presence of end amino acids in compositions of peptides the growth of liver and spleen tissues increased in cultures, obtained from old animals. Peptides increased the index of growth area of these tissues in 16-19% and combined of amino acids and peptides increased these values on 41-45%. Peptides and its combined with asparagine equally stimulated vessel organotypic tissue cultures, obtained from young and old animals growth on 61-74%. It was not verified significant differences in the index of growth area in brain cortex, heart and liver of old animals after the adding amino acids and peptides in comparison with peptides. It is possible, that proliferative potential of these tissues is not sensitive to amino acids. Investigated combines of short peptides with its end amino acids opened the new perspectives for creation of new substances, which can stimulate brain, heart, spleen and liver function in pathology and aging.
One of the most priority in cell biology and molecular medicine directions is the study of mechanisms of epigenetic regulation of organs functions and tissues at the cellular level. Preservation of homeostasis and biological unity of the organism at the tissue, subcellular and cellular level is possible due to the action of proteins and peptides regulatory, implementing a multistage equilibrium state between various physiological processes such as proliferation, differentiation, or estimated the cell death. Numerous data described in this area of knowledge contribute to the understanding of the fundamental mechanisms of molecular and cellular homeostasis, exchange and reproduction of genetic information and allow extrapolating these conclusions to find innovative approaches to the diagnosis, prevention and treatment of diseases, as well as increasing life expectancy.
The use of short geroprotective peptides containing 2-4 amino acid residues can serve as a promising and scientifically confirmed experimental method of increasing the duration and quality of life. Peptides activate mRNA expression of genes for growth, development and differentiation of tobacco plant cell culture (Nicotiana tabacum) [1]. The mutant lines of Drosophila agnts 3 peptide improves locomotor activity and memory processes information by epigenetic effects on the expression of mRNA of genes limkl, rok, park. Mutations in these genes are key factors in the development of Parkinson’s disease. The introduction peptides into the culture of chicken retinal embryo cells activates the differentiation of different types of neurons and retinal pigment epithelium [2]. The greatest increase in animal life expectancy by 42.3% was observed with the introduction of the peptide. The effect peptides on expression of 15247 genes of mouse heart and brain was studied using DNA microchip technology [3]. Peptides induce neuronal differentiation of human tooth tissue stem cells (hPDLSCs) and are promising for study as modulators of neurogenesis [4]. Peptides specifically regulate the expression of a certain group of genes and the synthesis of the corresponding proteins. In old monkeys (Macaca mulatta) after injection of peptides of an epiphysis normalization of level of melatonin and cortisol in blood is revealed [3]. The use of peptides in various experimental models led to an increase / decrease in the expression of mRNA genes and normalization of the synthesis of proteins encoded by these genes, which correlated with increased functions of various organs and tissues and increased life expectancy.
It is known that 20 encoded amino acids, which are structural elements of proteins and peptides, regulate the activity of various organs and tissues [5]. As the concentration of extracellular glutamine decreases, cell susceptibility to Fas-mediated programmed cell death increases [6]. This effect may also be one of the causes of skeletal muscle dysfunction [7]. The literature describes data on the regulation of proliferation and programmed cell death under the influence of the amino acid arginine. The introduction of an enzyme that reduces the level of arginine due to enzymatic degradation (arginase) into the culture of normal cells contributed to the arrest of the cell cycle in phases G0 and G1 with its subsequent normalization. It should be noted that in immortalized cell culture, the ability of cells to proliferate was not restored when arginine was administered [8]. Leucine, an amino acid with branched side radicals, increased DNA synthesis and hepatocyte proliferation in concentrations ranging from 10-5 to 10-3 M. Moreover, leucine increased the activity of the enzyme S6-kinase 1 (S6K1) and eukaryotic initiation factor (eIF4E) [9]. We have previously shown that encoded amino acids activate or inhibit cell proliferation and programmed cell death in animal tissue explants of different ages [10].
The aim of the work was to study the combined effect of short peptides and terminal amino acids in the structure of these peptides on the development of explants of cerebral cortex, heart, liver, spleen and blood vessels of animals of different ages.
Material and methods of research
The study used more than 700 explants of tissues of the spleen, liver, heart, cerebral cortex, 3-month (young) and 20-month (old) male Wistar rats from the collection of laboratory mammals of different taxonomic affiliation vivarium Institute of physiology. The isolated pieces of tissue were dispersed into smaller fragments of 1 mm3 size. These fragments were cultured in Petri dishes in a nutrient medium composed of 35% Needle medium, 35% Hanks solution, 25% fetal bovine serum. In cell culture medium was added glucose (0.6 percent), insulin (0.5 units/ml) and the antibiotic gentamicin 100 u/ml. All the explants were divided into 3 groups: 1 first — control, with 3 ml of cell culture medium, without addition of peptides and amino acids, 2 second- there in the cell culture were injected in 3 ml of nutrient medium with the studied concentration of peptides and 3 third – there in cell culture were injected in 3 ml of nutrient medium with the studied concentration of peptides and amino acids. Explants were grown in a CO2 incubator at a temperature of 37 °C at 5% CO2 for 3 days. After that, the cell culture samples were studied under a phase-contrast microscope. Peptides and adding amino acids (Sigma, USA) was added to the cell culture medium at final concentrations of 05 ng/ml. To study the combined action of peptides and amino acids, amino acids contained at the ends of peptides were selected, since in preliminary experiments they had the greatest biological activity. On the 3rd day of cultivation, a growth zone appeared around the explants, which was formed by proliferating and evicting cells of fibroblast-like morphology. To assess the growth zone of tissue explants we used a numerical indicator-the area index of the Explant growth zone (Index EGZ). The average value of EGZ for 15 explants was calculated. EGZ was calculated as the ratio of the difference in the area of the entire Explant, together with the peripheral growth zone, minus the Central zone, to the area of the Central zone of the Explant. EGZ in the group «control» took equal to 100%. EGZ in the study group, with the addition of biologically active substances, expressed as a percentage of the control. The significance of differences in the groups was assessed by the Student’s test criterion in Statistica 7.0. Differences between the groups were considered statistically significant at a significance level of p <0.05.
Results and discussion
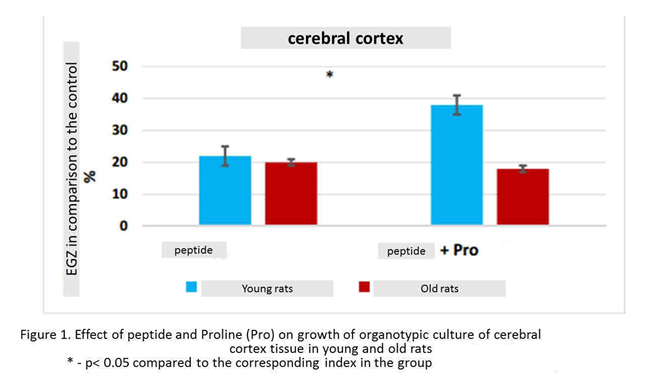
Effect of combined action of peptides and amino acids included in their composition on organotypic tissue cultures of young animals. Peptide increased the growth of cortical explants in 22% (n=15, p<0.05) compared to this indicator in the control group (n=14). Peptide and amino acid Proline increased the number of explants of the cerebral cortex by 38% (n=15, p<0.05) compared to this indicator in the «control» group. The effect of the combined action of peptide and Proline on the growth of organotypic tissue culture of the cerebral cortex of young rats was greater by 16% than the action of one peptide (figure. 1).
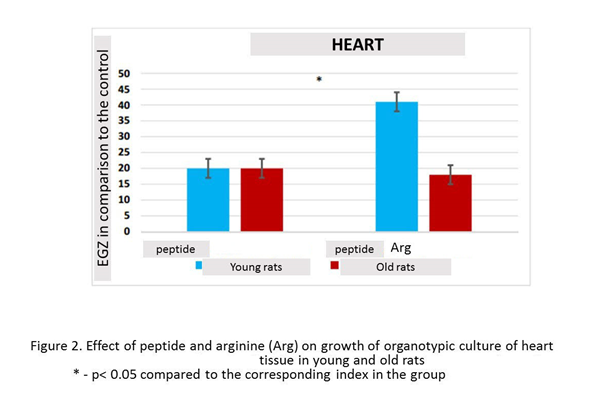
In the presence of peptide, the growth of organotypic culture of heart tissue was stimulated in 20% (n=13, p<0.05), compared with this indicator in the control group (n=15). Culturing of heart fragments in the presence peptide and arginine revealed a statistically significant increase in Explant EGZ by 41% (n=13, p <0.05) compared to this indicator in the control group. The effect of the combined action peptide and arginine on the growth of organotypic culture of heart tissue in young rats was greater in 21% than the action of one peptide (figure 2).
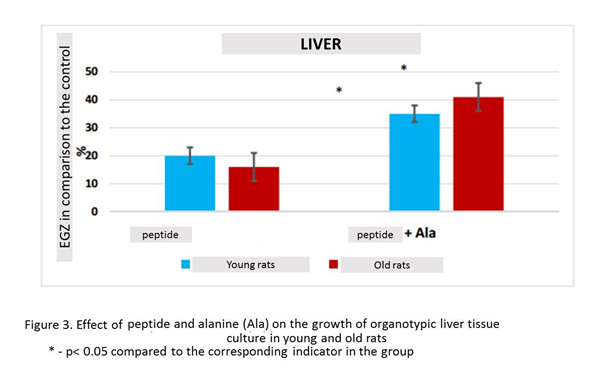
Peptide stimulated the growth of liver explants in 20% (n=15, p <0.05) compared to this indicator in the control group (n=13). When liver fragments were cultured in the presence peptide and alanine, there was a statistically significant increase in Explant EGZ in 35% (n=14, p <0.05) compared to this indicator in the control group. The effect of the combined action peptide and alanine on the growth of organotypic culture of liver tissue in young rats was 15% greater than the effect of one peptide (figure 3).
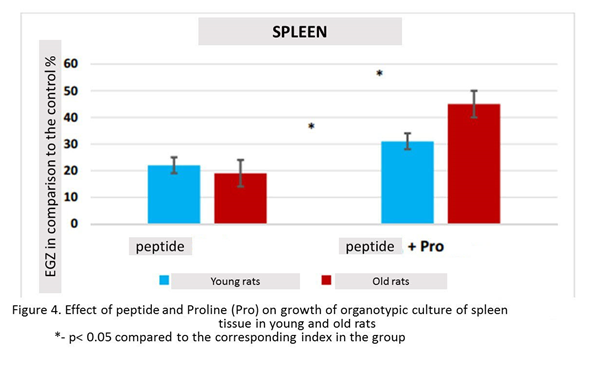
In the presence of the peptide, the growth of spleen organotypic culture was stimulated in 22% (n=13, p<0.05) compared to this indicator in the control group (n=13). The culturing of spleen fragments in the presence of Glu-Asp-Pro peptide and Proline revealed a statistically significant increase in Explant EGZ in 31% (n=12, p <0.05) compared to this indicator in the control group. The effect of the combined action peptide and Proline on the growth of organotypic culture of spleen tissue in young rats was greater by 9% than the effect of one peptide (figure 4).
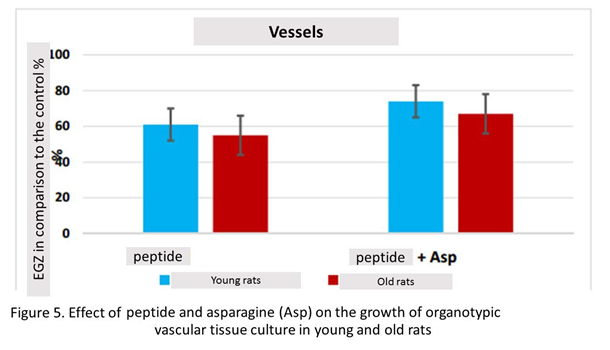
Peptide stimulated the growth of vascular explants by 61% (n=15, p<0.05) compared to this indicator in the control group (n=13). When vascular fragments were cultured in the presence peptide and asparagine, there was a statistically significant increase in Explant EGZ by 74% (n=12, p <0.05) compared to this indicator in the control group. The effects of the combined action peptide and asparagine on the growth of organotypic culture of vascular tissue in young rats did not differ significantly from the action of one peptide (Figure 5).
The effect of combined action of peptides and amino acids included in their composition on organotypic tissue cultures of old animals. Peptide stimulated the growth of cortical explants by 20% (n=16, p <0.05) compared to this indicator in the control group (n=15). When culturing fragments of the cerebral cortex in the presence peptide and Proline, there was a statistically significant increase in the number of explants by 18% (n=16, p <0.05) compared to this indicator in the control group. Thus, peptide and the combination of this peptide with Proline had the same stimulating effect on the growth of explants of the cerebral cortex of old animals (Fig. 1).
In the presence peptide, the growth of organotypic culture of heart tissue was stimulated by 21% (n=17, p <0.05) compared to this indicator in the control group (n=14). Culturing of heart fragments in the presence peptide and arginine revealed a statistically significant increase in Explant EGZ by 19% (n=16, p <0.05) compared to this indicator in the control group. Combination peptide with arginine had the same stimulating effect on the growth of heart explants in old rats (Fig. 2).
Peptide stimulated the growth of liver explants by 16% (n=18, p <0.05) compared to this indicator in the control group (n=15). When liver fragments were cultured in the presence peptide and alanine, there was a statistically significant increase in Explant EGZ by 41% (n=18, p <0.05) compared to this indicator in the control group. The increase in EGZ with the combined action of tetrapeptide and alanine exceeded EGZ with the action peptide by 25% (Fig. 3).
In the presence peptide spleen tissue growth was stimulated by 19% (n=15, p <0.05) compared to this indicator in the control group (n=15). The culturing of spleen fragments in the presence peptide and Proline revealed a statistically significant increase in Explant by 45% (n=15, p <0.05) compared to this indicator in the control group. The increase in IP with the combined action of Tripeptide and Proline exceeded IP with the action of the peptide by 24% (figure. 4).
Peptide stimulated the growth of vascular explants by 55% (n=16, p <0.05) compared to this indicator in the control group (n=16). When vascular fragments were cultured in the presence peptide and asparagine, there was a statistically significant increase in Explant by 67% (n=15, p <0.05) compared to this indicator in the control group. Thus, peptide and the combination of this peptide with asparagine had a stimulating effect on the growth of vascular explants of old animals, which did not differ statistically (figure. 5).
Conclusion
In the presence of terminal amino acids contained in the peptides, enhanced stimulating effect of peptides on the growth of explants of the cerebral cortex, spleen, liver and heart of young animals. Under the action of peptides, the growth zone of these tissues increased by 20-22%, whereas with the combined action of peptides and amino acids, this index increased to 31-41%.
In the presence of terminal amino acids contained in the short peptides, the stimulating effect of peptides on the growth of organotypic cultures of liver and spleen tissues of old rats increases. Under the action of peptides, the growth zone of these tissues increased by 16-19%, whereas with the combined action of peptides and amino acids, this index increased to 41-45%. It can be assumed that the simultaneous effect peptides and their terminal amino acid on the liver and spleen tissue of old rats is the summation of the effects of these two biologically active molecules. At the same time, in organotypic cultures of the cerebral cortex, heart and blood vessels of old animals, there were no differences in the effect on the area of tissue growth zone when amino acids were added to peptides. This may be due to the fact that the tissues of the cerebral cortex, blood vessels and heart are less susceptible to additional stimulation of their proliferative potential under the action of amino acids.
In organotypic cultures of vascular tissue of young and old animals peptide and its combination with asparagine equally stimulated the growth of explants by 61-74%. Probably, the absence of differences between the action peptide and its combination with amino acid on the growth of organotypic culture of vascular tissues is due to the initially high biological activity of the peptide.
The stimulating effect of combinations of short peptides with amino acids may be due to the fact that short peptides can penetrate the cytoplasm and the nucleus of the cell and bind to DNA. At the same time, encoded amino acids have the ability to regulate specific genes at transcriptional and translational levels, which contributes to the regulation of cell metabolism [11; 12].
The studied combinations of short peptides with terminal amino acids open prospects for the creation of new drugs that stimulate the function of tissues of the cerebral cortex, spleen, liver and heart in pathological processes, including those associated with age.
Literature
- Fedoreeva L. I., Dilovarov T. A., Ashapkin V. V., Martirosyan, Yu. TS., Khavinson V.Kh, Kharchenko P. N., Vanyushin B. F. Short exogenous peptides regulate gene expression families CLE, KNOX1 and GRF y Nicotiana tabacum // Biochemistry. 2017. T. 82. Vol.4. P. 700-709.
- Khavinson V. H., Pronyaeva V. E., Linkova N. S., Trofimova S. V. Peptidergic regulation of retinal embryonic cell differentiation / / Cell technologies in biology and medicine 2013. № 1. P. 57-60.
- Anisimov V.N., Khavinson V.K. Peptide bioregulation of aging: results and prospects. Biogerontology. 2010. Vol. 11. No 2. P. 139-149.
- Caputi S., Trubiani O., Bruna S., Trofimova S., Diomede F., Linkova N., Diatlova A., Khavinson V Effect of short peptides on neuronal differentiation of stem cells. International Journal of Immunapathology and Farmacology. 2019. Vol. 33. P. 1-12.
- Bourgoin-Voillard S., Goron A., Seve M., Moinard C. Regulation of the proteome by amino acids. Proteomics. 2016. Vol. 16. No 5. P. 831-846.
- Oehler R., Roth E. Regulative capacity of glutamine. Curr. Opin. Clin. Nutr. Metab. Care. 2003. Vol. 6. No 3. P. 277-282.
- Roth E., Oehler R. Hypothesis: Muscular glutamine deficiency in sepsis—a necessary step for a hibernation-like state? Nutrition. 2010. Vol. 26. No 5. P. 571-574.
- Philip R., Campbell E., Wheatley D.N. Arginine deprivation, growth inhibition and tumour cell death: 2. Enzymatic degradation of arginine in normal and malignant cell cultures. Brit. J. Cancer. 2003. Vol. 88. No 4. P. 613-623.
- Kimura M., Ogihara M. Effects of branched-chain amino acids on DNA synthesis and proliferation in primary cultures of adult rat hepatocytes. Eur. J. Pharmacol. 2005. Vol. 510. No 3. P. 167-180.
- Chalisova N. I., Kontsevaya E. A., Linkova N. S., Pronyaeva V. E., Chervyakova N. A., Umnov R. S., Benberin V. V., Khavinson V. Kh. Biological activity of amino acids in organotypic tissue cultures / / Cell technologies in biology and medicine.2013. № 2. P. 224-228.
- Brasse-Lagnel C., Lavoinne A., Husson A. Control of mammalian gene expression by amino acids, especially glutamine. Febs J. 2009. Vol. 276. P. 1826-1844.
12. Bruhat Y, Cherasse Y., Maurin A. ATF2 is required for amino acid-regulated transcription by orchestrating specific histone acetylation. Nucleic Acids Res. 2007. Vol. 35. P. 1312-1321.




