Peptide IPH-AEN contains a low molecular weight peptide, has chondroprotective and osteoprotective properties and a normalizing effect on cartilage and bone tissue.
Experimental studies have shown that the peptide IPH-AEN regulates metabolic processes in chondrocytes and osteocytes, increases the reserve capacity of the body, which suggests the effectiveness of the peptide IPH-AEN to normalize the functions and restore the cartilaginous and skeletal systems in human disorders of various origins.
Thus, the aim of the present research was to study chondroprotective, osteoprotective and other properties of the peptide.
Clinical characteristics of patients
Clinical studies of the peptide IPH-AEN were conducted in 88 people with various injuries of the knee joint aged 28 to 60 years (average age was 39.7±1.2 years).
Inclusion criteria: patients with I-II degree knee chondropathy, knee osteoarthritis 1-2 degree.
Exclusion criteria: acute diseases, patients with decompensated pathology, patients with exacerbation of chronic diseases.
To assess the effectiveness of the dose of 100 µg (n=88 people) for the studied peptides, we conducted studies on the efficacy of peptides at a dose of 50 µg (n= 86 people) and 150 µg (n=87 people). The dose of 100 µg per day for peptides is chosen as used due to the fact that according to the research of «St. Petersburg Institute of Bioregulation and gerontology» the effective dosage begins with 100 µg, and the increase in the dosage does not affect the degree of effectiveness [Khavinson V. H., Linkova N. S. 2013].
Study characteristic
IPH-AEN peptide was administered orally according to the scheme: 1 capsule (100 µg peptide) 1 time per day for 30 days, then 30 days break — and repeat the same course for another 30 days, again break 30 days — and the third course for 30 days. The total course was 6 months (3 courses per 30 days and 3 breaks per 30 days).
The effectiveness of the improved administrated scheme for such patients with IPH-AEN peptide was evaluated after 3 and 6 months. The initial data obtained before the study were selected for the control values.
The efficacy of IPH-AEN peptide was evaluated based on the level of calmodulin in buccal epithelium.
Obtaining of samples of buccal epithelium from the oral cavity (mucosa of the cheek) was carried out no earlier than 4 hours after a meal, with a sterile cytometry with synthetic bristles. After taking the scraping, it is placed by shaking in a sterile disposable tube «Eppendorf» with saline solution.
Cytological smears were prepared by liquid Cytology using Novoprep automated system (NRS, France).
Verification of the studied signal molecules expression is associated with the use of hybridization methods in situ of specific matrix RNA (mRNA) and electron microscopy, with the use of antibodies to active molecules. Buccal epithelium specimens were placed on poly-L-lysine-coated slides (Sigma). Primary and secondary antibodies — monoclonal mouse anti-human antibodies to the studied signal molecules (Novocastra) were used for immunocytochemical studies. Visualization of the immunohistochemical reaction was performed using avidin complex with biotinylated peroxidase (ABC-kit), followed by the manifestation of horseradish peroxidase diaminobenzidine (Novocastra).
The technique of visualization of the expression of all antibodies was carried out according to the following scheme.
- For the blockade of endogenous peroxidase, a container with glasses was placed in a 3% aqueous solution of peroxide for 10 minutes.
- Cleaning in 2 changes of distilled water for 5 minutes each.
- Cleaning in 2 shifts of Tris-NaCl buffer pH 7.6 for 5 minutes each.
- Incubation with normal nonimmune serum for 30 minutes at room temperature. Application of 50-100 µl of normal blocking serum on the glass and placing the glasses in a damp chamber.
- Incubation in a thermostat with primary (specific) antibodies (+37°C, 1 hour).
- Cleaning in 2 shifts of Tris-NaCl buffer pH 7.6 for 5 minutes each.
- Incubation with secondary biotinylated antibodies for 30 minutes at room temperature.
- Cleaning in 2 shifts of Tris-NaCl buffer pH 7.6 for 5 minutes each.
- Incubation with ABC-kit for 30 minutes at room temperature.
- Cleaning in 2 shifts of Tris-NaCl buffer pH 7.6 for 5 minutes each.
- Detection of horseradish peroxidase by diaminobenzidine (the solution was prepared according to the instructions attached to the set of Novocastra). The reaction was controlled under a microscope.
- Cleaning in 2 changes of distilled water for 5 minutes each.
- Cleaning with water
- Dehydration — exposure for 3 minutes in each of the following solutions: alcohol 96%, alcohol 96%, carboxyla, xylene, xylol.
- Visualization using confocal microscope Olympus FluoView FV1000 at 400 magnification.
Calmodulin is a small, acidic, highly conservative calcium-binding protein, a representative of the EF-hand protein superfamily.
Calmodulin is found in the cytoplasm of all eukaryotic cells, which distinguishes it from other calcium-binding proteins. The protein molecule consists of two globular lobes (domains) separated by a Central spiral hinge. Each globular fraction has two Ca2+-binding sites containing the spiral-loop-spiral motif (EF-hand). Calmodulin itself does not show enzymatic activity, but is an integral subunit of a number of enzymes (protein kinases, proteinphosphatases, phosphodiesterases, enzymes of muscle mobility). It binds and activates more than 40 targets. The concentration of calmodulin in the cell varies within 5-10 microns and is about half the concentration of all Self-binding proteins.
A decrease in this protein is detected when cartilage tissue is damaged and leads to a deterioration in regeneration and repair.
Norm of calmodulin level (Anti — CalEF-hand antibody) in buccal epithelium (25-60 years): 7.8 – 14.6 conventional units (unit).
The effectiveness of the IPH-AEN peptide was also evaluated on the basis of subjective sensations (using VAS – visual analog scale) and the hyaline cartilage of the knee joints was assessed by ultragraphy on the Samsung SonoAce R3 using a high-frequency linear sensor with a frequency of 7.5 — 10.0 MHz. The structure and thickness of the hyaline cartilage of the femoral condyles of the injured knee joint were evaluated with the calculation of the index of degenerative thinning of the cartilage (IDI) by the ratio of the thickness of the hyaline cartilage of the loaded surface to the thickness of the cartilage of the posterior surface of the femoral condyle. The degree of structural changes of the cartilage was assessed according to the ICRS classification (International Repair Society), modified for ultrasonic diagnostic, which identified 4 degrees of degenerative changes in cartilage: 0 (normal cartilage), 1 degree (increased echogenicity, moderately irregular contour, ordinary thickness), stage 2 (increased echogenicity, heterogeneous structure, irregular contour, reducing the thickness of the cartilage up to 50% of IDA of 0.5-0.8), 3 degree (increased echogenicity, heterogeneous structure, markedly irregular contour with eroded areas, thinning of the cartilage more than 50%, less than 0.5 IDIH), 4 degree (larger areas the lack of cartilage on the articular surface).
Statistical processing of results
Statistical analysis was carried out using the program STATISTICA 11. The statistical methods of data processing were based on the calculation of the mean absolute and relative values with the calculation of the average error; the significance of the differences between the two sets was estimated using the student t criterion (the difference was considered reliable at t>2, p<0.05).
Result of the study
The results of a clinical study of the IPH-AEN peptide using showed that patients after 3 months of using the peptide significantly improved subjective assessment of pain in 1.4 times (table 1).
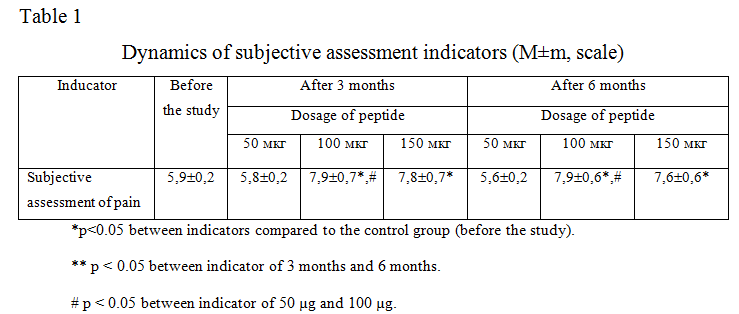
This confirms the fact that the use of the peptide IPH-AEN leads to a decrease in the inflammatory response and thus to a decrease the pain, observed in patients with degenerative — dystrophic lesions of bone and cartilage.
We have not found any significant differences between the indicators in the application of 100 mcg and 150 mcg according to all the studied parameters, which proves the fact that with an increase in the optimal dosage of the peptide used, the efficiency does not increase both after 3 months and after 6 months.
Also, we have not found significant differences between the indicators in the application of 50 mcg and before the study on all the studied parameters, which proves the fact that the effective optimal dosage for the peptide is 100 mcg.
The use of the peptide IPH-AEN increases the expression of the molecular marker of calmodulin to the upper limit of the norm. At the same time, at the beginning of the study, the level of calmodulin was reduced due to the presence of degenerative cartilage lesions in the studied patients (table 2).
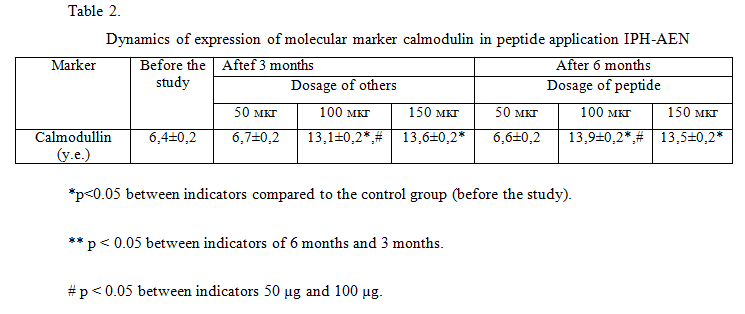
Thus, we have found that the use of the peptide IPH-AEN contributes to a significant improvement in the regeneration and repair of bone and cartilage tissue in degenerative lesions of bone and cartilage tissue, as well as counteracts degenerative processes in the cartilage tissue of the joints, thereby ensuring the prevention of joint diseases.
We have not found any significant differences between the indicators in the application of 100 mcg and 150 mcg according to all the studied parameters, which proves the fact that with an increase in the optimal dosage of the peptide used, the efficiency does not increase both after 3 months and after 6 months.
Also, we have not found significant differences between the indicators in the application of 50 mcg and before the study on all the studied parameters, which proves the fact that the effective optimal dosage for the peptide is 100 mcg.
The dynamics of the index of cartilage degenerative thinning has been shown in table 3.
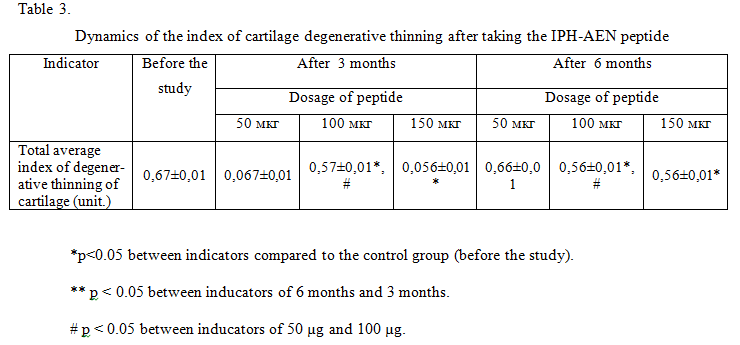
The use of the peptide IPH-AEN led to a positive dynamics of ultrasound: decreased echogenicity and become more clear contours of articular cartilage, decreased the rate of cartilage degenerative thinning in 0.9 times. These data indicate that the use of the peptide IPH-AEN leads to the normalization of the structure and thickness of the cartilage surface in degenerative – dystrophic lesions of bone and cartilage tissue, which is necessary to maintain the full functionality of the joints.
We have not found any significant differences between the indicators in the application of 100 mcg and 150 mcg in all the studied parameters, which proves the fact that with an increase in the optimal dosage of the peptide used, the efficiency does not increase neither after 3 months nor after 6 months.
Also, we have not found significant differences between the indicators in the application of 50 mcg and before the study in all the studied parameters, which proves the fact that the effective optimal dosage for the peptide is 100 mcg.
Conclusion
Thus, the use of the peptide IPH-AEN leads to a decrease in the inflammatory response and thus to a decrease in pain, observed in patients with degenerative and dystrophic lesions of cartilage.
The use of the peptide IPH-AEN contributes to a significant improvement in the regeneration and repair of bone and cartilage tissue in degenerative and dystrophic lesions of bone and cartilage tissue, and also counteracts degenerative processes in the cartilage tissue of the joints, thereby ensuring the prevention of joint diseases.
The use of the peptide IPH-AEN leads to the normalization of the structure and thickness of the cartilaginous surface in degenerative and dystrophic lesions of bone and cartilage tissue, which is necessary to maintain the full functionality of the joints.
The use of the peptide IPH-AEN is recommended as a dietary Supplement with a therapeutic and prophylactic purpose to normalize the functions of bone and cartilage.
Literature
- Zabello T.V., Miromanov A. M., Mirimanova N. A. Genetic aspects of osteoarthritis // Fundamental research – 2015. – № 1-9. – P. 1970-1976
- Linkova N. S., Drobantseva A. O., Orlova O. A., Kuznetsova E. P., Polyakova O. V., Kvetnoy I. M., Khinson V. Kh. Peptide regulation of functions of skin fibroblasts upon aging in vitro // Cell technologies in biology and medicine. 2016. — №1. – 40-44.
- Paltseva E. M., Polyakova V. O., Krolewski V. A. buccal cells. New approaches to molecular diagnostics of socially significant pathology. : Publishing: «N-L», 2015. 128 с. ISBN: 978-5-94869-176-3
- Havinson V. Kh. Peptide regulation of aging. SPb.: Science 2009. — 50 p.
- Trzeciak T. MicroRNAs: Important Epigenetic Regulators in Osteoarthritis / T. Trzeciak, M. Czarny-Ratajczak // Curr. Genomics. – 2014. – № 6. – P. 481–484.
- Tsezou A. Osteoarthritis year in review 2014: genetics and genomics // Osteoarthritis Cartilage. – 2014. – № 12. – P. 2017–2024.




